Christmas Newsletter

As the year draws to a close, we take pride in reflecting on the diverse projects we’ve supported across the life sciences and healthcare.
From academic research to industry collaborations, our work has centred on one shared goal: turning data into meaningful insights that improve patient care and inform better decisions.
This year, our team has:
- Supported randomised clinical trials for both industry and academic partners
- Advanced radiotherapy research through decision‑support tools
- Delivered Real‑World Evidence (RWE) studies to strengthen regulatory strategies, value dossiers and reimbursement pathways
- Partnered with MedTech companies to refine diagnostic tools in oncology
- Created visualisations that transform complex RWE into clear, actionable insights
We are grateful for the trust placed in us and look forward to continuing to support innovative projects in the year ahead. Contact us on PGEgY2xhc3MgPSAibF9tYWlsIGxfbmV3X3dpbmRvdyIgaHJlZj0ibWFpbHRvOmluZm9AZXBpc3RhdC5zZSIgdGFyZ2V0PV9ibGFuaz5pbmZvQGVwaXN0YXQuc2U8L2E+ for any questions or proposals!
Recent publications
During 2025 Epistat has contributed to no less than 16 scientific publications, out of which three in the highly estimated journal The Lancet. The articles span the areas of cardiovascular disease and oncology, with many interesting findings. Below you can read more about our publication in The Lancet Oncology, and our website will guide you to all of our published work.

Routine scans may not boost survival in melanoma follow‑up
Our CEO Anders Berglund has co‑authored a major study on follow‑up care for high‑risk melanoma, published in The Lancet Oncology. The interim findings from the TRIM trial show that routine whole‑body imaging after surgery does not improve survival outcomes compared with standard physical examinations alone. While results so far suggest no added benefit, the trial will continue to full completion to confirm long‑term effects. Click the links below for further articles in The Lancet.
The Lancet Digital Health, October 2025
The Lancet Diabetes & Endocrinology, October 2025

VizCan – Visualizing Cancer Outcomes
Sweden has unique health registers with data ready to be explored. In the VizCan tool, Epistat and Vision Zero Cancer have assembled cancer‑related data to make it easier to access and interpret. Epistat’s Biostatistician Hanna Vikman is responsible for the visualization, making complex cancer outcomes clearer for both experts and citizens.
VizCan highlights regional differences in cancer incidence, survival, and mortality. The tool has been presented to major patient associations, who greatly appreciated its clarity. By making outcomes easy to understand, VizCan informs the public and supports doctors, policymakers, and decision‑makers. Explore the data yourself at vizcan.se!

Tailored treatments for common disease
Using advanced statistical modelling, our Data Scientist Hampus Hållberg has contributed to the development of a programme that will optimise treatment for a widespread disease while reducing the risk of adverse side effects.
The programme can anticipate which side effects are most likely to occur with a given treatment plan. This enables clinicians to adjust treatment proactively, leading to safer therapies and improved patient outcomes. This exemplifies Epistat’s commitment to supporting clinicians with innovative tools that strengthen evidence-based healthcare.

Target Trial Emulation clarifies chemotherapy timing in lung cancer
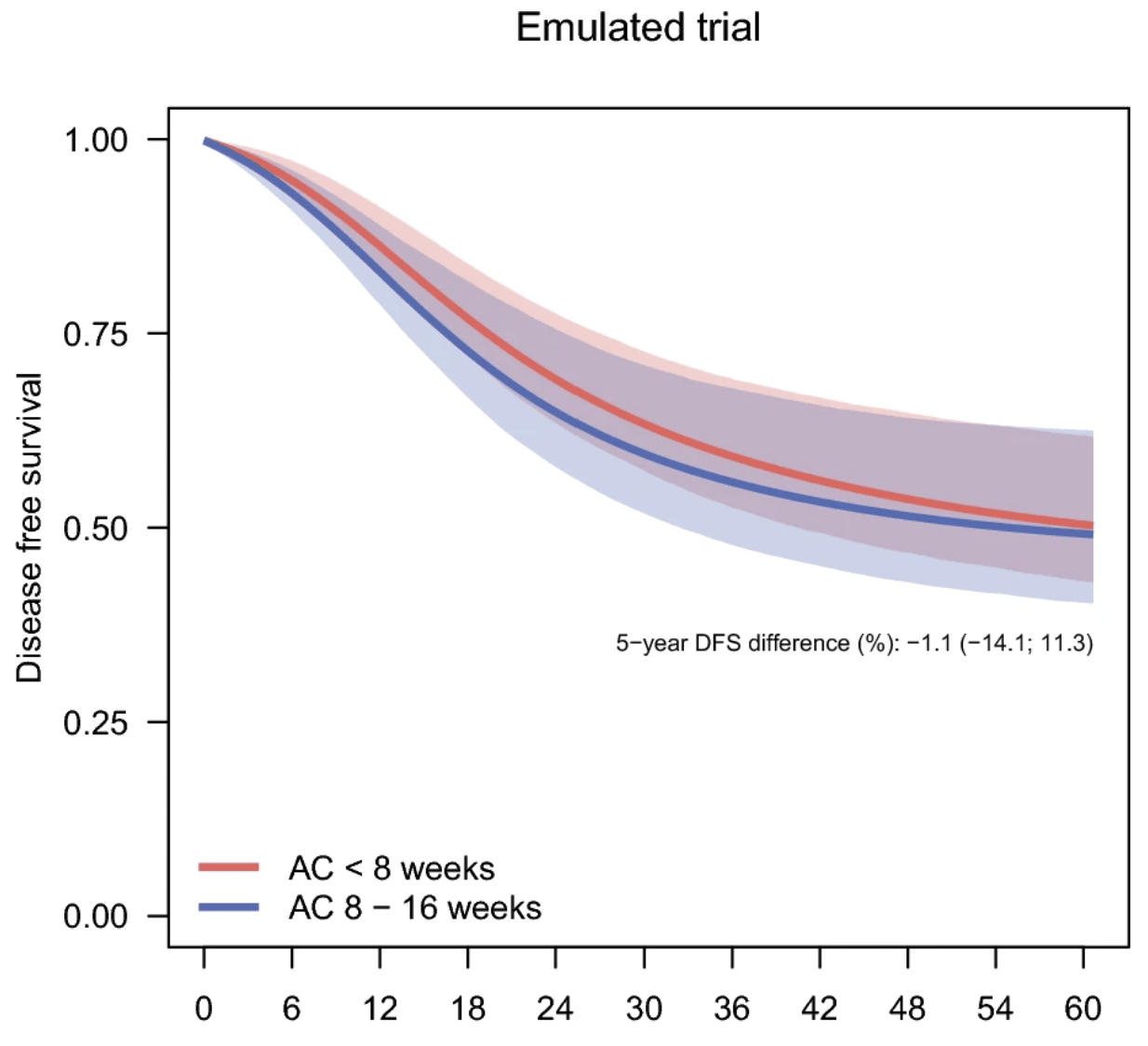
A recently published project, led by Senior Biostatistician Erik Lampa, examined the timing of chemotherapy after surgery for non‑small cell lung cancer, using data from the Swedish Lung Cancer Registry. The study, appearing in Annals of Surgical Oncology, applied a target trial emulation approach to address methodological challenges such as immortal time bias, which can arise when treatment start dates are misaligned with diagnosis or surgery.
By using modern causal inference methods, including the Clone-Censor-Weight technique, Erik and colleagues emulated a hypothetical randomised trial comparing patients who began adjuvant therapy before versus after the recommended 8‑week window. The results showed no evidence that later treatment initiation was linked to poorer survival, providing valuable insights into real‑world treatment patterns.
Advancing Diagnostic Support and Real-World Evidence
At the beginning of the year, Epistat’s Biostatistician Valentina Fermanelli contributed to a MedTech company’s study evaluating its diagnostic support software for pathologists. By comparing the software's results with diagnoses of experienced pathologists, the company confirmed a high level of accuracy. This highlights the software’s potential as a reliable aid in clinical decision‑making and demonstrates the strength of combining statistical expertise with innovative technology.
More recently, Valentina has been closely involved in real‑world evidence projects based on the Swedish Lung Cancer Registry. The projects involved every stage, from drafting protocols and statistical analysis plans to programming and running analyses, highlighting the complexity of working with real‑world data and turning it into meaningful insights.

We hope you enjoyed the read
and wish you all a very Merry Christmas!

